Classification of matter pogil answers – Delving into the realm of matter classification, this comprehensive guide unravels the intricate tapestry of matter’s composition and properties. Embark on a journey to decipher the mysteries of pure substances, mixtures, elements, compounds, and molecules, while exploring the fascinating world of physical and chemical changes.
Prepare to unlock the secrets of stoichiometry, solutions, acids, bases, and pH, as we delve into the captivating world of chemistry.
Through engaging explanations and real-world examples, this guide illuminates the fundamental principles governing the behavior of matter. Discover the characteristics that define gases, solids, and liquids, and explore the transformative power of phase diagrams in predicting matter’s state. Witness the practical applications of chemistry in diverse fields, from medicine to engineering and agriculture, gaining a profound appreciation for its transformative impact on our world.
1. Matter Classification

Matter, anything that takes up space and can be weighed, can exist in three primary states: solid, liquid, and gas. Each state has distinct characteristics:
- Solidshave a fixed shape and volume, with particles tightly packed together in a regular arrangement.
- Liquidshave a fixed volume but no definite shape, conforming to the shape of their container. Particles in liquids are closely spaced but can move more freely.
- Gaseshave no definite shape or volume, expanding to fill their container. Gas particles are widely spaced and move rapidly.
Examples of substances in each state include:
- Solid: ice
- Liquid: water
- Gas: air
The state of matter is influenced by factors such as temperature and pressure.
2. Pure Substances and Mixtures

Pure substancesare composed of only one type of atom or molecule. They can be either elements or compounds.
Mixturesare combinations of two or more different substances. They can be homogeneous (uniform throughout) or heterogeneous (non-uniform).
Examples of pure substances include:
- Element: gold
- Compound: water
Examples of mixtures include:
- Homogeneous: saltwater
- Heterogeneous: sand in water
3. Elements, Compounds, and Molecules: Classification Of Matter Pogil Answers
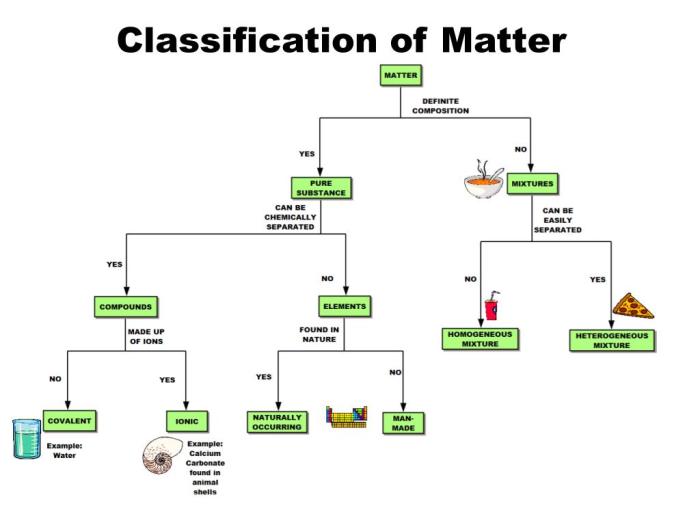
Elementsare pure substances that cannot be broken down into simpler substances by chemical means. They are represented by the symbols in the periodic table.
Compoundsare pure substances composed of two or more different elements chemically combined in fixed proportions. They have different properties from their constituent elements.
Moleculesare the smallest units of a compound that retain its chemical properties. They are composed of two or more atoms held together by chemical bonds.
Examples of elements include:
- Hydrogen (H)
- Oxygen (O)
Examples of compounds include:
- Water (H 2O)
- Carbon dioxide (CO 2)
Examples of molecules include:
- Water molecule (H 2O)
- Carbon dioxide molecule (CO 2)
FAQ Insights
What are the three primary states of matter?
The three primary states of matter are solid, liquid, and gas.
What is the difference between a pure substance and a mixture?
A pure substance is a substance that has a constant composition throughout, while a mixture is a combination of two or more substances that are not chemically bonded.
What are the four types of chemical reactions?
The four types of chemical reactions are synthesis, decomposition, single displacement, and double displacement.