Draw a six carbon alkyne that can exist as diastereomers – Introducing the fascinating world of diastereomers, draw a six-carbon alkyne that can exist as diastereomers. Diastereomers, non-mirror-image stereoisomers, arise when a molecule possesses multiple stereocenters. This article delves into the structural intricacies and properties of six-carbon alkynes capable of exhibiting diastereoisomerism.
Delving deeper, we explore the stereochemistry of six-carbon alkynes, examining the various types of stereoisomers and their three-dimensional representations using Newman projections. The influence of stereochemistry on physical and chemical properties is also brought to light.
Diastereomers of Six-Carbon Alkynes
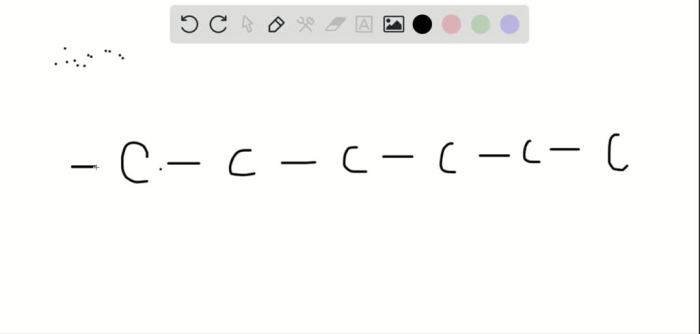
Diastereomers are stereoisomers that are not mirror images of each other. They have the same molecular formula and connectivity, but differ in the spatial arrangement of their atoms. A six-carbon alkyne can exist as diastereomers if it has at least one chiral center.
The structural feature that enables a six-carbon alkyne to exist as diastereomers is the presence of a double bond with two different substituents. This double bond can exist in two different orientations, resulting in two different diastereomers.
The following figure shows the two possible diastereomers of a six-carbon alkyne:
- Diastereomer 1: The two substituents on the double bond are on the same side of the molecule.
- Diastereomer 2: The two substituents on the double bond are on opposite sides of the molecule.
Stereochemistry of Six-Carbon Alkynes
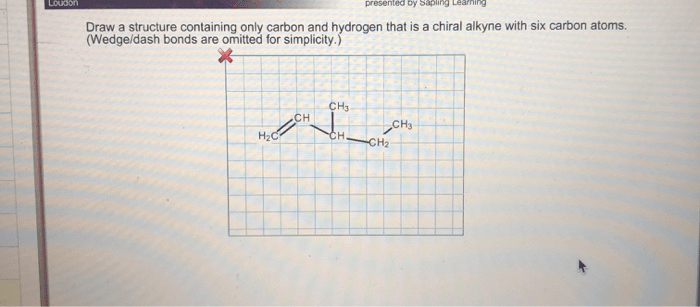
The stereochemistry of a six-carbon alkyne refers to the three-dimensional arrangement of its atoms. Six-carbon alkynes can exist as different types of stereoisomers, including enantiomers, diastereomers, and meso compounds.
Newman projections are a convenient way to represent the three-dimensional structures of six-carbon alkynes. Newman projections show the molecule as if it is being viewed down the C-C bond. The front carbon atom is represented by a dot, and the back carbon atom is represented by a circle.
The stereochemistry of a six-carbon alkyne can affect its physical and chemical properties. For example, the melting point and boiling point of a six-carbon alkyne can vary depending on its stereochemistry.
Methods for Synthesizing Diastereomeric Six-Carbon Alkynes
There are several different synthetic methods that can be used to prepare diastereomeric six-carbon alkynes. One common method is the alkyne coupling reaction. In this reaction, two alkyne molecules are coupled together using a palladium catalyst.
Another method for synthesizing diastereomeric six-carbon alkynes is the alkyne metathesis reaction. In this reaction, an alkyne molecule is metathesized with another alkyne molecule using a ruthenium catalyst.
The choice of synthetic method depends on the desired diastereomer and the starting materials that are available.
Applications of Diastereomeric Six-Carbon Alkynes

Diastereomeric six-carbon alkynes have a variety of applications in different fields, including pharmaceuticals, materials science, and catalysis.
In pharmaceuticals, diastereomeric six-carbon alkynes are used as building blocks for the synthesis of complex natural products. For example, the anti-cancer drug Taxol contains a six-carbon alkyne moiety.
In materials science, diastereomeric six-carbon alkynes are used as monomers for the synthesis of polymers. These polymers can have unique properties, such as high strength and toughness.
In catalysis, diastereomeric six-carbon alkynes are used as ligands for transition metal catalysts. These catalysts can be used to perform a variety of reactions, including hydrogenation, oxidation, and cycloaddition.
FAQs: Draw A Six Carbon Alkyne That Can Exist As Diastereomers
What are diastereomers?
Diastereomers are stereoisomers that are not mirror images of each other.
How many diastereomers are possible for a six-carbon alkyne?
Two diastereomers are possible for a six-carbon alkyne.
What are the applications of diastereomeric six-carbon alkynes?
Diastereomeric six-carbon alkynes have applications in pharmaceuticals, materials science, and catalysis.